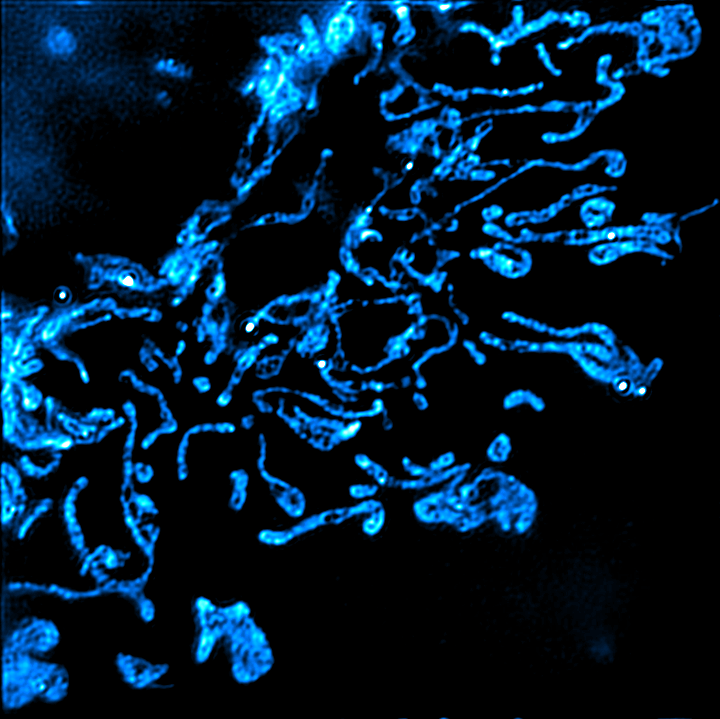
Mitochondria are famously known as the “powerhouse of the cell;’ however, this phrase does not do them justice, as mitochondria regulate many other important functions, such as apoptosis, lipid metabolism, calcium signaling, etc. Perhaps the most interesting aspect of mitochondrial biology is the fact that they originated from bacteria, and still harbor molecules that resemble bacterial molecules (such as their own DNA, mtDNA). Intriguingly, one function of mitochondria is the regulation of innate immunity, which enables cells to detect invasion by pathogens and/or cellular damage. Mitochondria trigger innate immune pathways by releasing mitochondrial molecules such as mtDNA, and many important innate immune signaling proteins localize either close to, or directly on, mitochondria. The major focus of the lab is understanding how this ancient bacterium regulates the innate immunity. Our primary experimental approaches include microscopy and live cell imaging, which allow us to observe the release of mitochondrial components (such as mtDNA), contact sites between mitochondria and other organelles, and the dynamics of mitochondria. Our goal in understanding these processes is mechanistic information that may lead to new treatments to ameliorate pathological inflammation underlying diseases (such as autoimmune diseases, neurodegenerative diseases, etc.). Projects fall within the following themes:
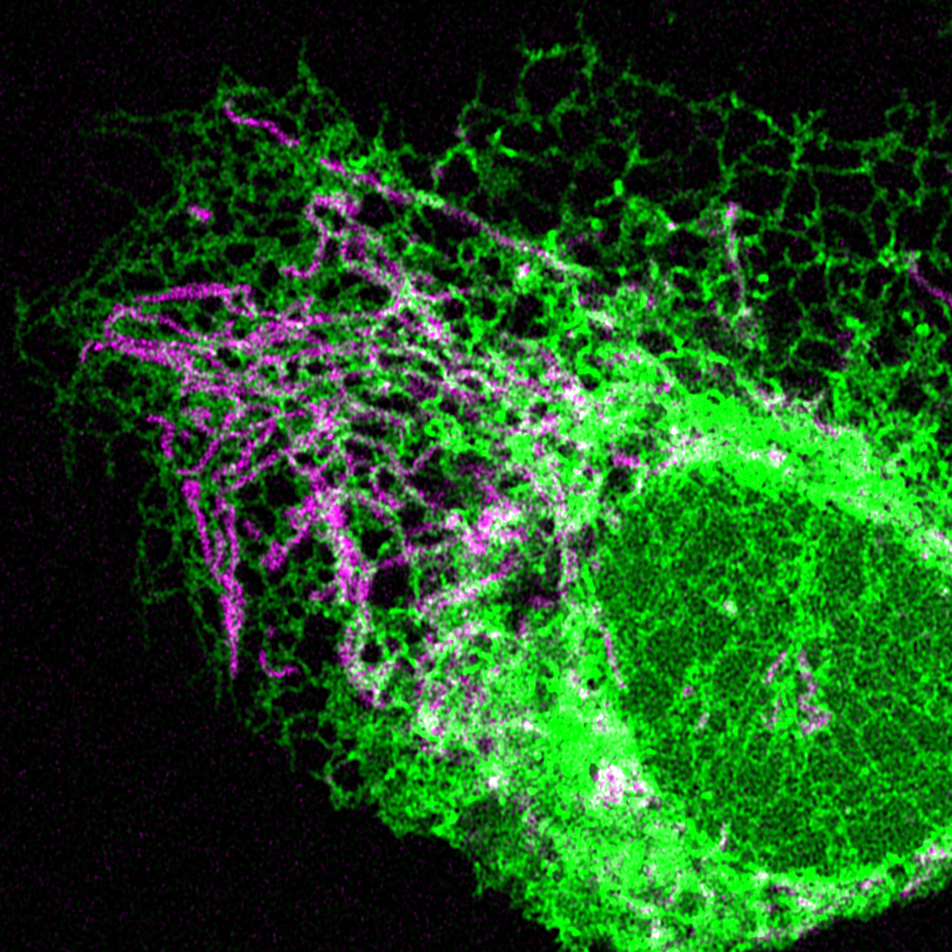
Contact sites between mitochondria and the ER are important signaling platforms for innate immunity, as key innate immunity proteins localize to these contacts. In addition to innate immunity, mitochondria/ER contacts also regulate numerous processes critical to mitochondrial homeostasis, including mtDNA replication, mitochondrial morphology, lipid metabolism, calcium signaling, apoptosis, and autophagy. Therefore, our goal is to understand why innate immune signaling occurs near mitochondria, and how mitochondrial function is integrated with innate immunity at these contact sites.
Mitochondrial DNA activates innate immune pathways when released from mitochondria, contributing to pathological inflammation associated with a long and growing list of human diseases. We recently discovered that non-replicating mtDNA traffics through endosomes for degradation, and that this pathway enables mtDNA release when endosomes rupture and leak mtDNA into the cytosol. We are continuing our studies of the fundamental biology of this endosomal mtDNA disposal and release pathway, with the goal of understanding how mitochondria-endosome communication enable the escape of mtDNA from mitochondria.
Work from our lab and other labs has shown that HSV-1 attacks mtDNA during infection, which triggers mtDNA release via the endosomal pathway described above, indicating that mtDNA plays signaling roles during HSV-1 infection. Intriguingly, this is not limited to HSV-1, as several other viruses (including influenza, zika, dengue, etc.) have also been shown to attack mitochondria or mtDNA, resulting in mtDNA release. We are studying the roles of mtDNA release in the cellular warfare with viruses and testing whether mtDNA acts as an antiviral signaling molecule after its release. In the long term, we plan to test whether persistent mitochondria-driven antiviral signaling may underlie chronic diseases that are triggered by viral infection (such as ME/CFS, Long Covid, and neurodegenerative disease).
Mitochondrial health is intricately linked with cellular and organismal health and physiology. We are deeply interested in the fundamental cell biology of mitochondrial stress signaling. We aim to understand how the communication between mitochondria and other cellular organelles maintains proper cellular physiology, and how dysfunction of these pathways may lead to chronic disease.